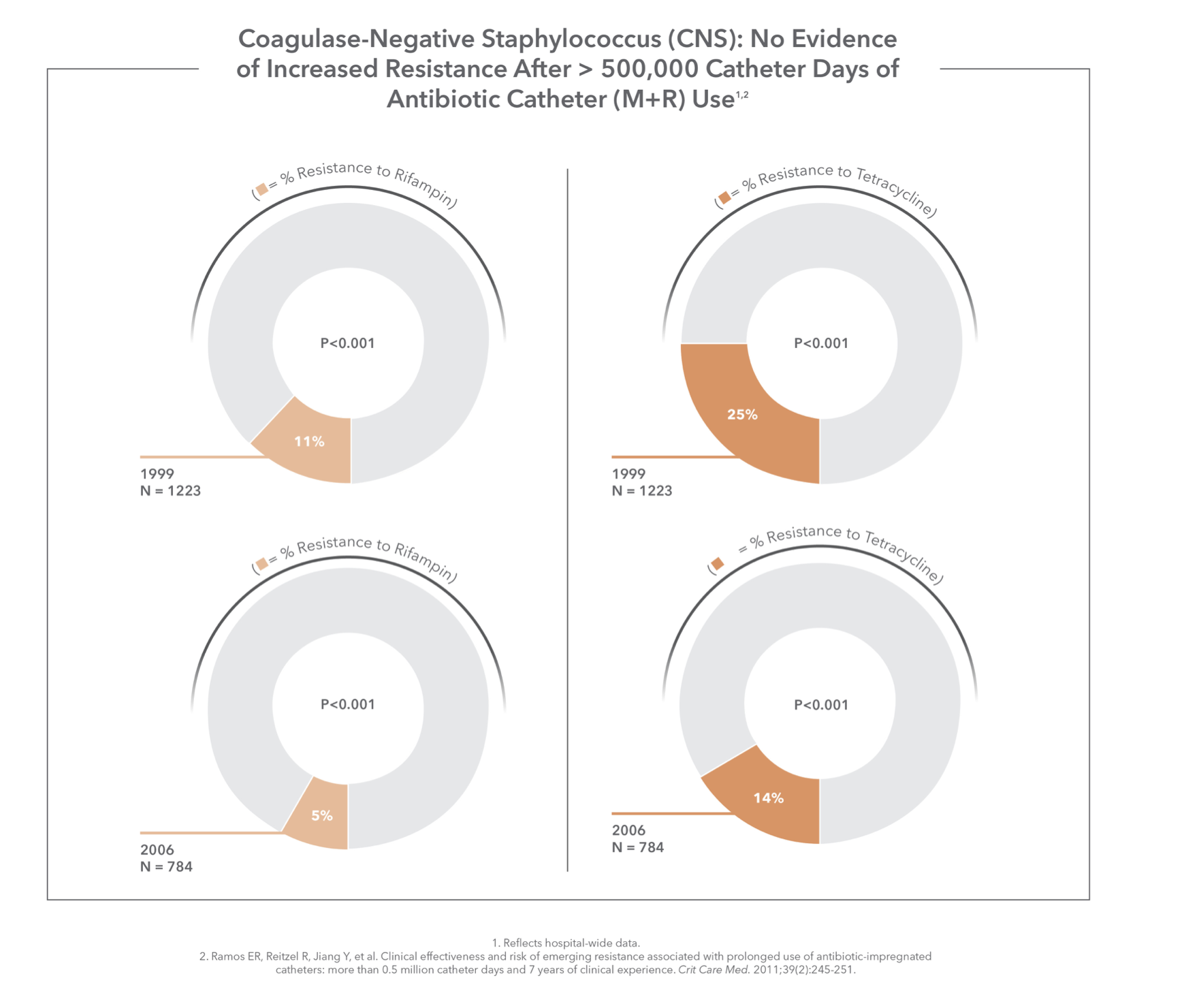
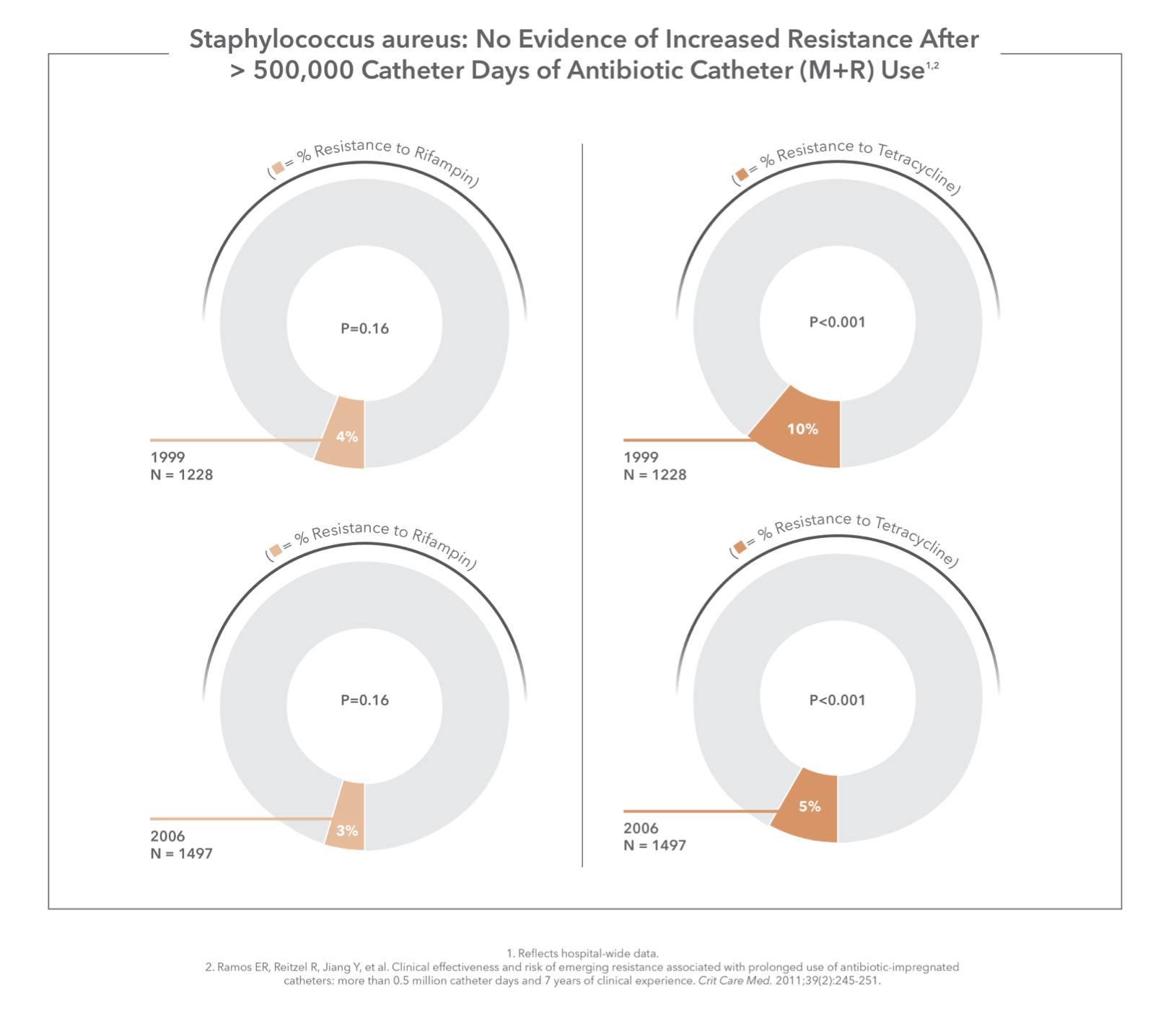
No Evidence of Increased Resistance

Five prospective studies have examined the susceptibility of clinical isolates to the development of antibiotic resistance from M+R CVC use. None of the studies showed any association between M+R and antibiotic resistance, nor did a 7-year study of over 500,000 catheter days.1